Today, the class spent the majority of the time going over how the velocity of individual gas molecule relates to states of the gas such as pressure or temperature.
During the first few moments of class, a program was used to simulate the behavior of gas molecules in an enclosed container. If one were to change any of the values assigned to the state variables of the gas, then the other values would change in relation to that altered value. We used the values given by this program to solve for the Ideal Gas Constant "R"
Afterward, we used the definition for concepts such as momentum and pressure to derive a series of equations, one of which being the root-mean-square speed of a gas molecule.
A demonstration was then performed in order to show the behavior of gas with a physical object manipulating its flow. Prior to the demonstration, the class was asked to predict whether the flame of the candle at the bottom of the cylinder would quickly lose oxygen and burn out or if it would continue to burn with the tube of paper held over the top of it. As it turns out, the tube created a channel for the heated air to escape, causing cooler air to flow down the sides and continue feeding the flame.
This then lead to a discussion of the adiabatic process. More equations were derived including the work done during an adiabatic process.
After this, we use a device that would serve to demonstrate the adiabatic process on an observable volume. A piece of paper was used to show that as the volume in the chamber gets smaller and more compact, the temperature increases, so much so that it eventually reaches the ignition point of the paper.
The final temperature inside the chamber was calculated using rough estimates.
Sunday, March 16, 2014
Mon. Week 2, March 3: The Ideal Gas Law
On this day, the class performed labs revolving around the ideal gas law. The first lab had us taking measurements that were later used to generate a graph that displays the linear relationship between temperature and volume.
The air inside the flask was heated while the attached syringe measured the increase in the air's volume.
After enough data points were taken, the information was used to generate a graph. However, the graph was not linear as was expected, possibly due to a bad measurement.
We got an estimated value for the coefficient of the equation V=kT by fitting a linear line to the generated graph. We determined that in order for the coefficient to make sense, the dimensions of the coefficient k must be m^3/K.
The next part of class consisted of attempting to take Boyle's law and Charles's law and relate them together in order to come up with a unified equation for the relationships between pressure, volume and temperature.
After unifying the gas equation, we performed an exercises in class that revolved around the real-world applications of the ideal gas law.
Lastly, the class was treated to a demonstration of the effects of the gas law on objects such as a balloon and marshmallow. The objects were placed in a vacuum chamber where air was sucked out, causing the gasses inside each object to undergo an increase in volume through expansion.
The air inside the flask was heated while the attached syringe measured the increase in the air's volume.
After enough data points were taken, the information was used to generate a graph. However, the graph was not linear as was expected, possibly due to a bad measurement.
We got an estimated value for the coefficient of the equation V=kT by fitting a linear line to the generated graph. We determined that in order for the coefficient to make sense, the dimensions of the coefficient k must be m^3/K.
The next part of class consisted of attempting to take Boyle's law and Charles's law and relate them together in order to come up with a unified equation for the relationships between pressure, volume and temperature.
After unifying the gas equation, we performed an exercises in class that revolved around the real-world applications of the ideal gas law.
Lastly, the class was treated to a demonstration of the effects of the gas law on objects such as a balloon and marshmallow. The objects were placed in a vacuum chamber where air was sucked out, causing the gasses inside each object to undergo an increase in volume through expansion.
Monday, March 3, 2014
Wed. Week 1, Feb. 26: Thermal expansion, latent heat and pressure relationships
Today, the class continued coverage on thermodynamics by performing specific experiments on thermal expansion, latent heat of a phase change and pressure relationships.
Thermal expansion was the first attempted lab. An aluminum tube pushed on a pulley as it expanded with added heat from steam traveling through the tube.
The degrees that the pullley was turned in degrees was measured and graphs were generated for both angle and temperature
The next experiment was the latent heat, and was accomplished by simply using a Styrofoam cup as a thermal insulator, an immersion heater and a temperature probe attached to logger pro.
In this experiment, we started off by predicting the shape of the temperature vs. time graph of ice water slowly being heated to its boiling point.
Afterward, the experiment was performed for the class, which showed that there was no flat region where the water still contained significant amounts of ice as was expected. This leads to the conclusion that the immersion heater was adding heat to the water faster than the ice could absorb it.
After that, the class collected their own data on an experiment for the latent heat of vaporization. All the values were collected to get a value for our measurement's uncertainty, which was then used to see if our measured values fell within range of the true value.
For the last part of lab, the class attempted to predict the relationship between pressure and several other values.
The relationship between pressure and volume proved to be curved instead of linear as expected, which may be due to the dynamics of the gas molecules at high pressure.
Thermal expansion was the first attempted lab. An aluminum tube pushed on a pulley as it expanded with added heat from steam traveling through the tube.
The degrees that the pullley was turned in degrees was measured and graphs were generated for both angle and temperature
The next experiment was the latent heat, and was accomplished by simply using a Styrofoam cup as a thermal insulator, an immersion heater and a temperature probe attached to logger pro.
In this experiment, we started off by predicting the shape of the temperature vs. time graph of ice water slowly being heated to its boiling point.
Afterward, the experiment was performed for the class, which showed that there was no flat region where the water still contained significant amounts of ice as was expected. This leads to the conclusion that the immersion heater was adding heat to the water faster than the ice could absorb it.
After that, the class collected their own data on an experiment for the latent heat of vaporization. All the values were collected to get a value for our measurement's uncertainty, which was then used to see if our measured values fell within range of the true value.
For the last part of lab, the class attempted to predict the relationship between pressure and several other values.
The relationship between pressure and volume proved to be curved instead of linear as expected, which may be due to the dynamics of the gas molecules at high pressure.
Sunday, March 2, 2014
Mon. Week 1, Feb. 24: Thermal Conductivity and Heat Capacity
This day's lab experiments consisted of taking measurements to verify the values such as thermal conductivity and heat capacity of different materials.
Prior to the start of our experiments, we began our study of thermodynamics by developing a conversion factor between the Fahrenheit and Celsius temperature scales.
The equipment we used to take measurements for both experiments was Logger Pro with temperature probes. In the thermal conductivity lab, we used the aluminum can submerged in water. The water outside was of a significantly different temperature than the water outside the can and the temp. probes were used to measure the change in heat for both liquids. For the heat capacity lab, the Styrofoam cup was used as a thermal insulator so we could get accurate measurements for the amount of temperature change that took place for an amount of time that the immersion heater was on.
The resulting graph for the thermal conductivity lab displays non-linear relationship between temperature and time, which indicates that the heat exchange decreases when the temperature disparity between the two liquids reduces.
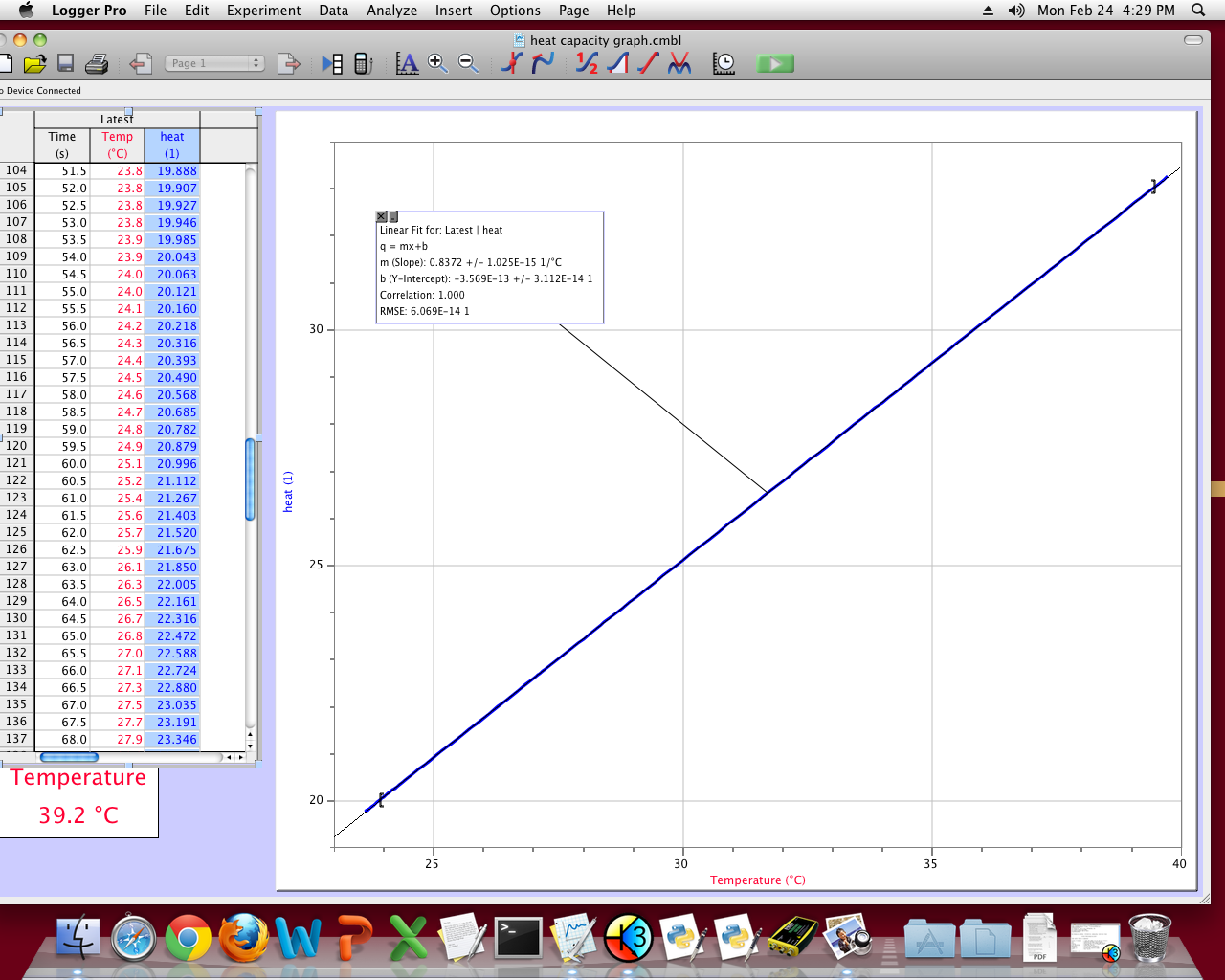
The resulting graph for the heat capacity lab shows a linear relationship between added heat and temperature change. The slope for this graph does not match with the known value of specific heat capacity for water because specific heat takes mass into account, and the displayed graph does not.
Prior to the start of our experiments, we began our study of thermodynamics by developing a conversion factor between the Fahrenheit and Celsius temperature scales.
The equipment we used to take measurements for both experiments was Logger Pro with temperature probes. In the thermal conductivity lab, we used the aluminum can submerged in water. The water outside was of a significantly different temperature than the water outside the can and the temp. probes were used to measure the change in heat for both liquids. For the heat capacity lab, the Styrofoam cup was used as a thermal insulator so we could get accurate measurements for the amount of temperature change that took place for an amount of time that the immersion heater was on.
The resulting graph for the thermal conductivity lab displays non-linear relationship between temperature and time, which indicates that the heat exchange decreases when the temperature disparity between the two liquids reduces.
The resulting graph for the heat capacity lab shows a linear relationship between added heat and temperature change. The slope for this graph does not match with the known value of specific heat capacity for water because specific heat takes mass into account, and the displayed graph does not.
Subscribe to:
Comments (Atom)